Study, 006-Earth Atmosphere
¡á Summary

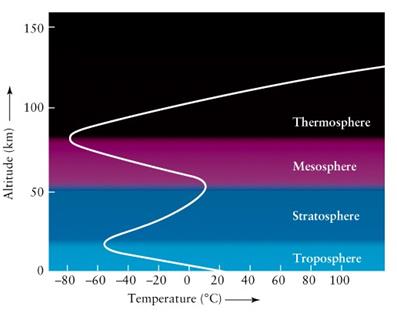
- Troposphere (´ë·ù±Ç)
- Stratosphere (¼ºÃþ±Ç)
- Mesosphere (Áß°£±Ç)
- Thermosphere (¿±Ç)
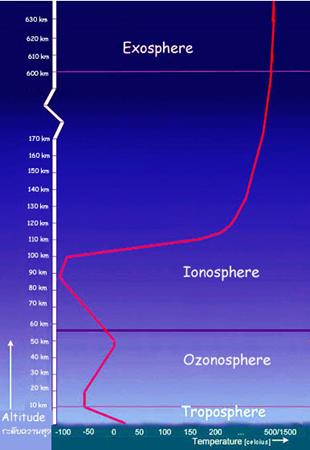
- Exosphere (¿Ü±Ç)
¡á Troposphere:
- Air heated by
sun-warmed ground
- Warm closest to
ground/sea level
- Heat from bottom causes
convection
- ±â»óÇö»ó, °ø±â 75% ¼öÁõ±â 99% ºÐÆ÷, ±Ø Áö¿ª 0-7km, Àûµµ Áö¿ª 0-20km, ÁßÀ§µµ Áö¿ª 0-17km
- °ø±â±¸¼º: 78.1% Áú¼Ò, 21.0% »ê¼Ò, 0.9% ±âŸ ±âü
- Planetary boundary
layer: Áö¸é°úÀÇ ¸¶Âû·ÂÀ¸·Î °ø±âÈ帧ÀÌ ¿µÇâÀ» ¹ÞÀ½. 0.2-2km
- Tropause: ´ë·ù±Ç/¼ºÃþ±Ç °æ°è¸é. ¿Âµµ°¡ °íµµ¿¡ µû¶ó °¨¼ÒÇÏÁö ¾Ê°í ÀÏÁ¤
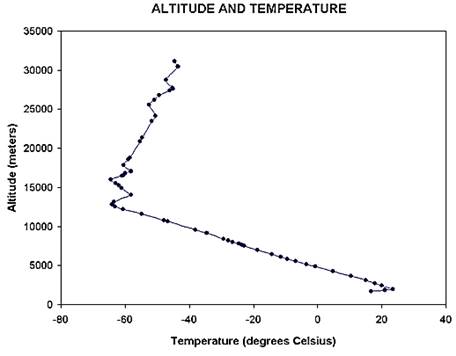
- ¿Âµµ°¡ ³ôÀÌ¿¡ µû¶ó ¼±ÇüÀûÀ¸·Î °¨¼Ò: -60¨¬C at 12 km
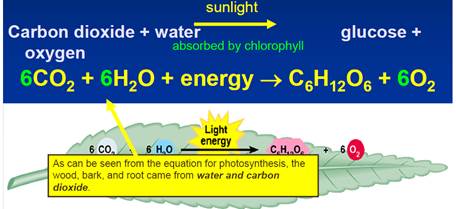
- Oxygen comes from
biological processes
- Life on Earth for 3.8
billion years (Earth is 4.5 billion years old)
- Humidity, pressure,
temperature decease with altitude
¡á Tropopause
- Boundary between the
troposphere and the stratosphere
- The jet stream is just
below the tropopause: 400 km/h speed
¡á Stratosphere
- Ozone layer (at 00 km)
blocks the sun's UV light
- If the sun's UV light
is not blocked, you will get a bad burn in 10 seconds.
- Ozone generated naturally
in stratosphere by sunlight
- Temperature increases
with altitude: no convection, stable air (no mixing, no turbulence)
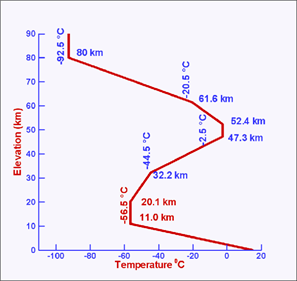
- Maximum height: 45-50
km
- Air is very dry (little
water vapor): no cloud except in polar regions in winter at 15-25 km and at
-78¨¬C
- Air is very thin.
¡á Stratopause
- Boundary between the
stratosphere and the mesosphere
¡á Mesosphere
- Altitude: 50-85 km
- Meteors
¡á Thermosphere
- Altitude: 85-690 km
- Fades off into thin
interplanetary gas
- UV radiation ionizes
the gas in the thermosphere creating the ionosphere
- AM and short-wave radio
waves bounce off of ionosphere
- Auroa
- Space Shuttle
¡á Exosphere
- Altitude: 690-10,000 km
¡á Gas pressure of atmosphere
- Each gas molecule
attracted to a planet by gravity
- Gas molecules hit each
other
- Balance of forces
determines atmospheric pressure
- Highest at the surface
and decreases smoothly as altitude increases
- On Earth, atmospheric
pressure decreases by a factor of 2 every 5.5km
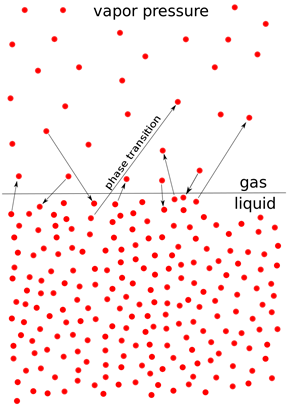
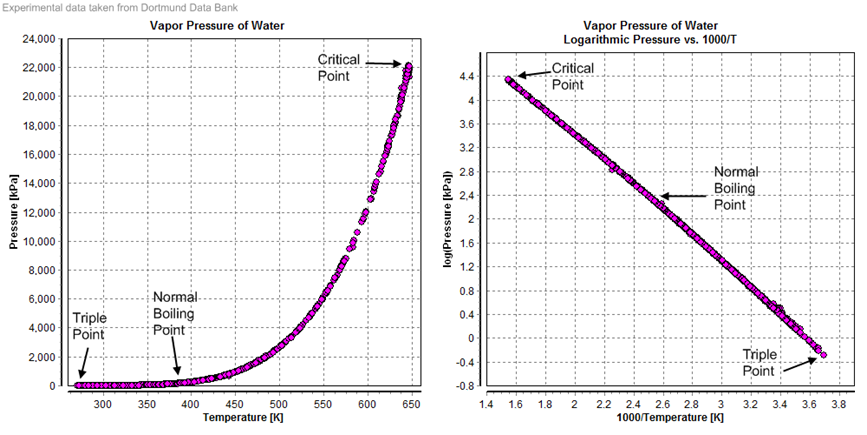
¡á Vapor
pressure (= equilibrium vapor pressure):
-
Pressure exhibited by vapor present above a liquid surface
-
Pressure exerted by a vapor in thermodynamic equilibrium with its condensed
phases (solid or liquid) at a given temperature in a closed system.
- An
indication of a liquid's evaporation rate.
- High
vapor pressure = volatile.
- Vapor
pressure vs temperature: nonlinear, Clausius-Clapeyron relation
-
Boiling point: at a given atmospheric pressure, vapor pressure = ambient
atmospheric pressure
-
Partial pressure: h=0m, T=20¨¬C, saturated with water vapor, water vapor 2.3kPa,
nitrogen 78kPa, oxygen 21kPa, 0.9kPa argon ¡æ a total of 102.2kPa standard atmospheric pressure
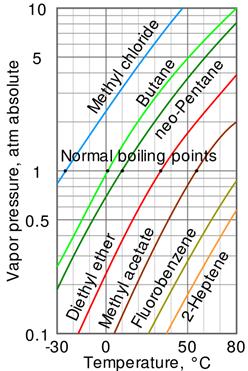
-
Antoine equation: vapor pressure vs temperature
![]()
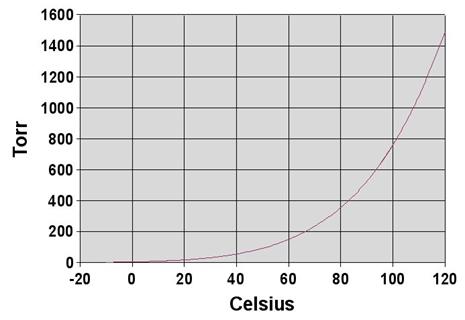
- Boiling point of water:
![]()
1 Torr = 1/760 atm =
133.3 Pa
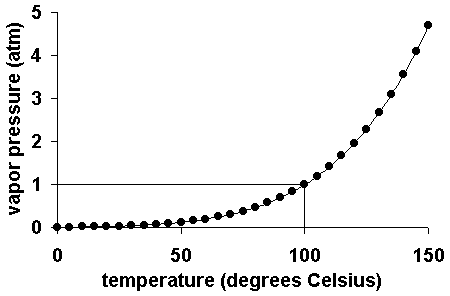
- Saturation vapor
pressure = equilibrium vapor pressure
- Equilibrium vapor
pressure of water
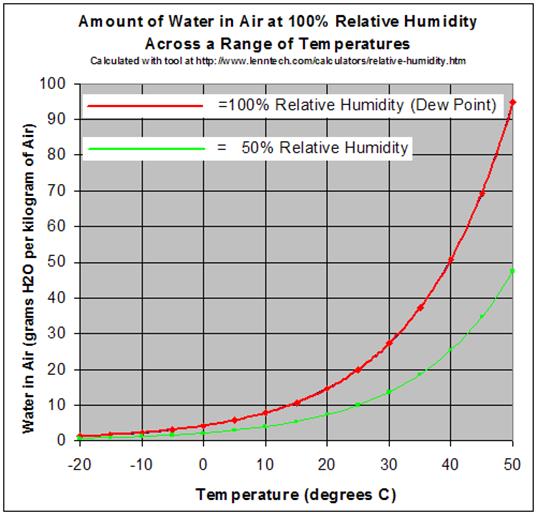
- RH (relative humidity) =
(partial pressure of water vapor) / (equilibrium vapor pressure of water)
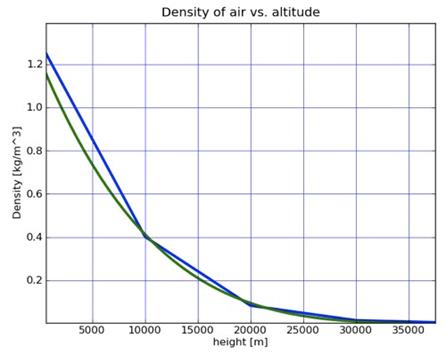
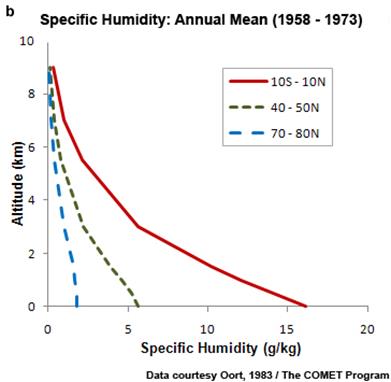
¡á Physical data by altitude
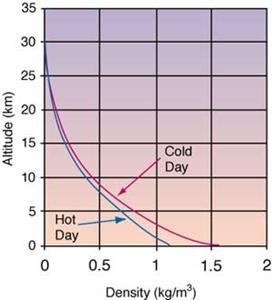
- Air density
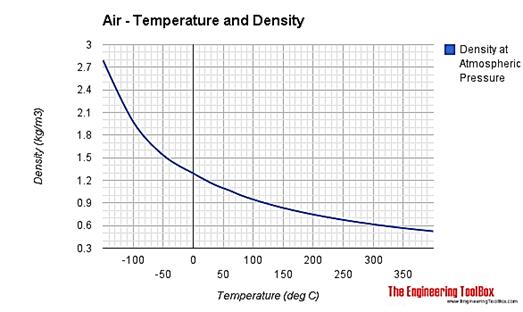
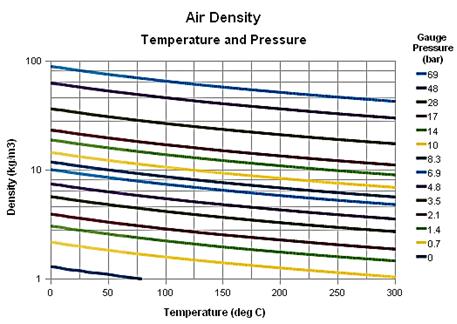
- Temperature vs altitude
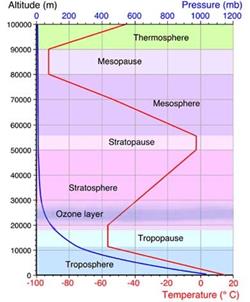
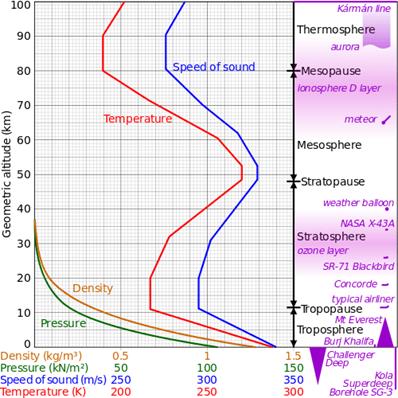
[Ref]
International standard
atmosphere: cavcar